Help patients adhere weight-bearing protocols to enhance bone healing & prevent fixation failure. These protocols reduce complications that lead to reoperations, generating additional revenue for practices and savings for payers.

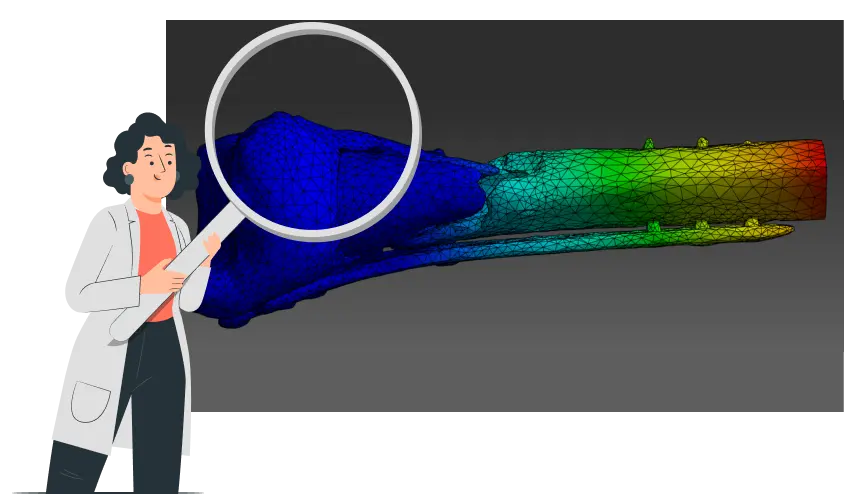
1. *CBM create 3D model from a post-op CT scan & calculate what load help the bone heal without compromising fixation
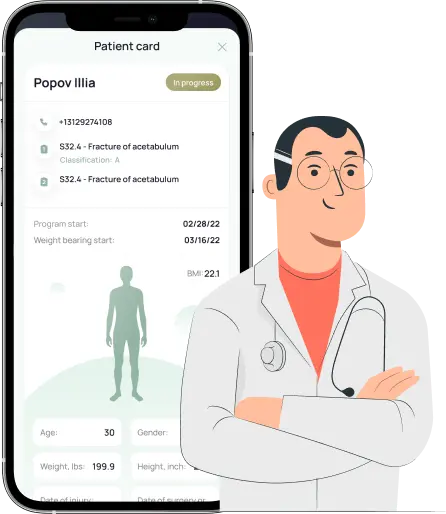
2. via *CBM app prescribe personalized load for the patient & remotely monitors compliance
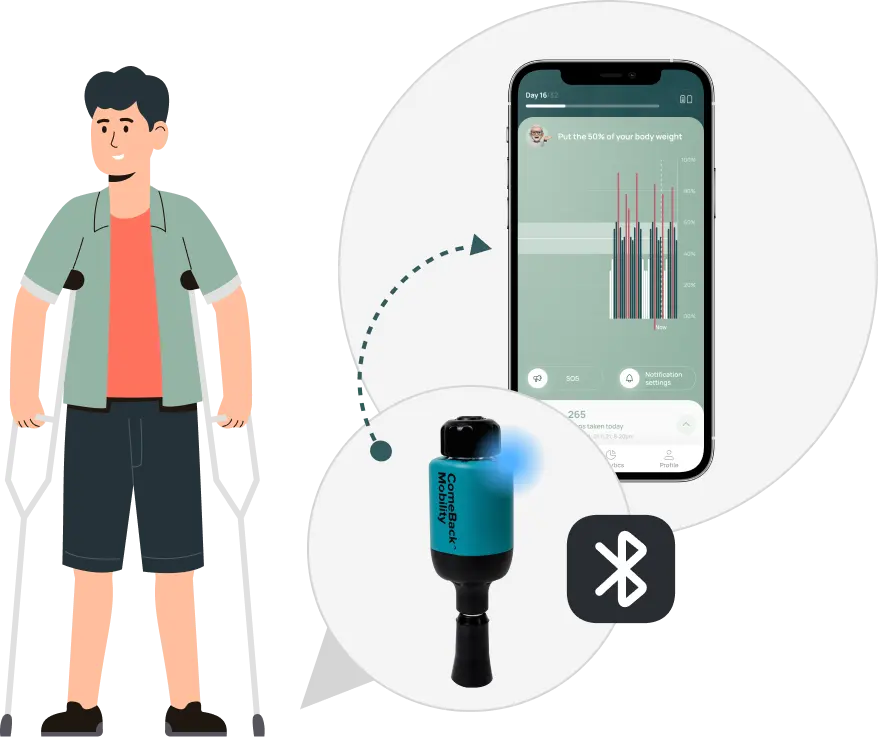
3. Patient use *CBM app & FDA cleared Crutch Tips to follow the prescribed loading plan.

1. *CBM create 3D model from a post-op CT scan & calculate what load help the bone heal without compromising fixation

2. via *CBM app prescribe personalized load for the patient & remotely monitors compliance

3. Patient use *CBM app & FDA cleared Crutch Tips to follow the prescribed loading plan.
in their rehabilitation
axial load
step data
of pain and swelling
in their rehabilitation
axial load
step data
of pain and swelling
Weight-bearing as tolerated is not always safe!
Individualized Determination of the Mechanical Fracture Environment After Tibial Exchange Nailing—A Simulation-Based Feasibility Study
Benedikt J. Braun, Marcel Orth, Stefan Diebels, Kerstin Wickert, Annchristin Andres, Joshua Gawlitza, Arno Bücker, Tim Pohlemann, Michael Roland (2021)
Weight-bearing as tolerated is not always safe!
Case:
A 55-year-old woman broke a leg, 7 weeks after surgery, the metal piece used to fix her bone broke, and her leg broke again
Why It Happen?
Researchers found that too much load during walking caused the implant to fail
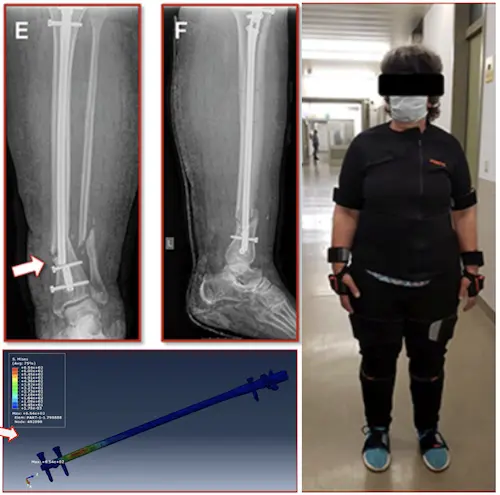
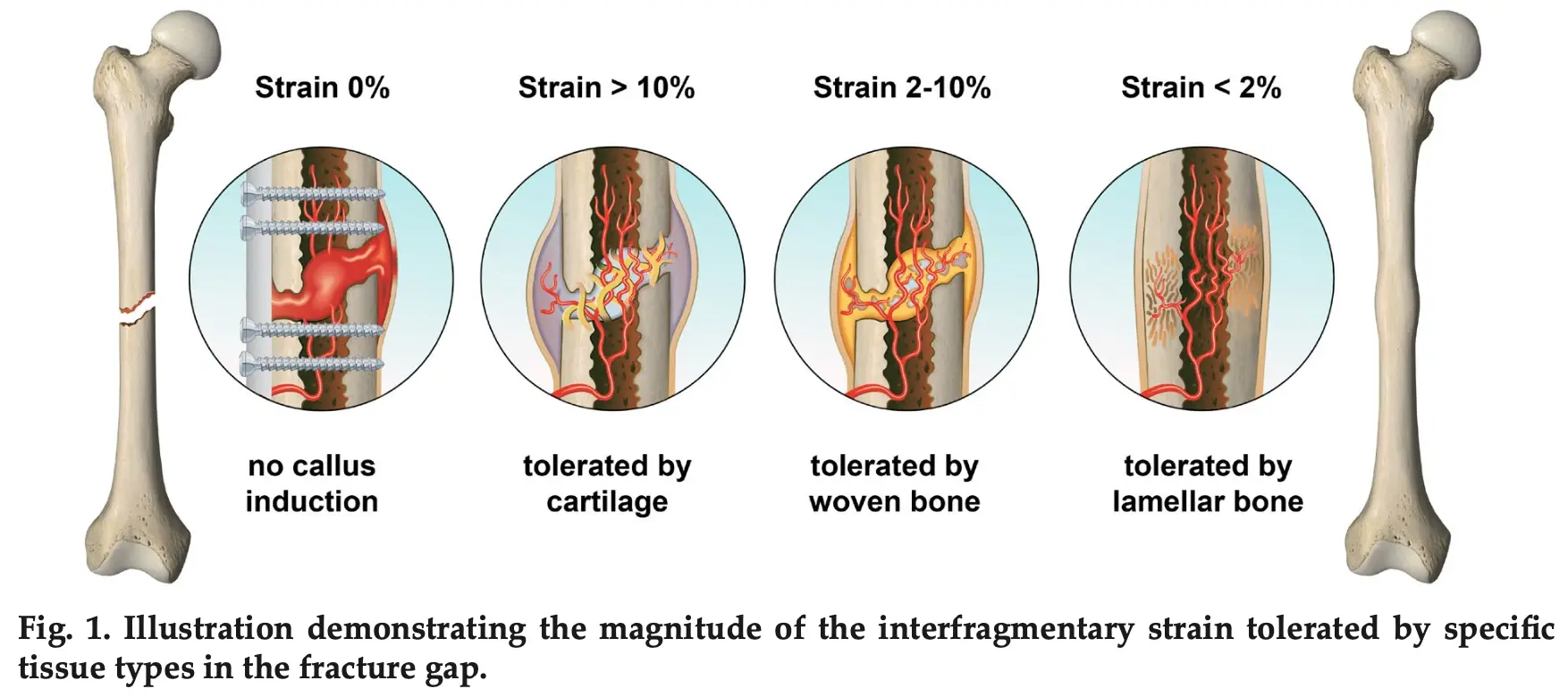
Applying Right Amount Of Strain Improves Healing!
If strain goes beyond certain limits – it slows down the healing process!
Controlled Mechanical Stimulation in the Treatment of Tibial Fractures
John Kenwright, Ph.D., F.R.C.S., And Allen E. Goodship,H.D., D.V.Sc., M.R.C.V.S., (1988)
Applying Right Amount Of Strain Improves Healing!
If strain goes beyond certain limits – it slows down the healing process!
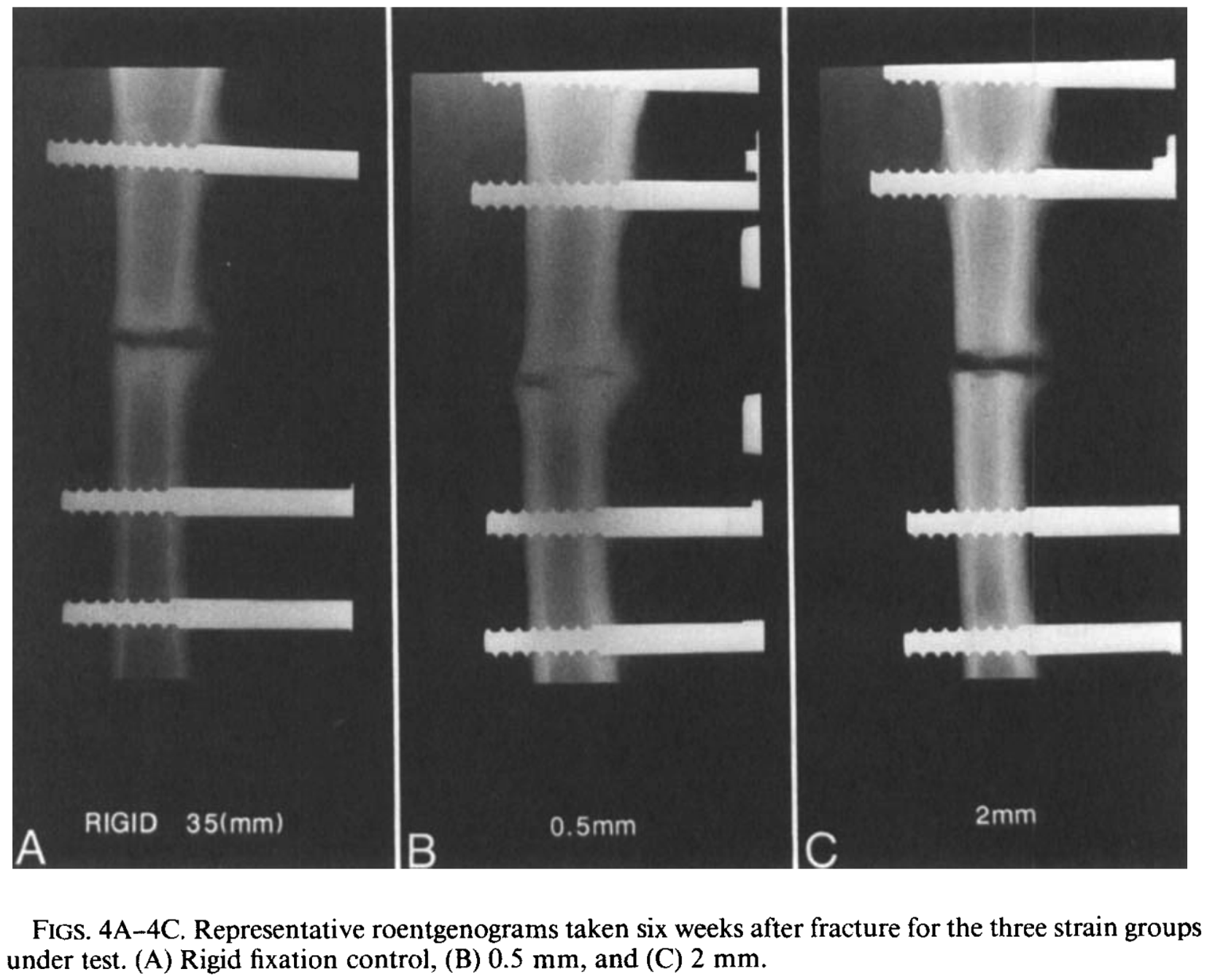
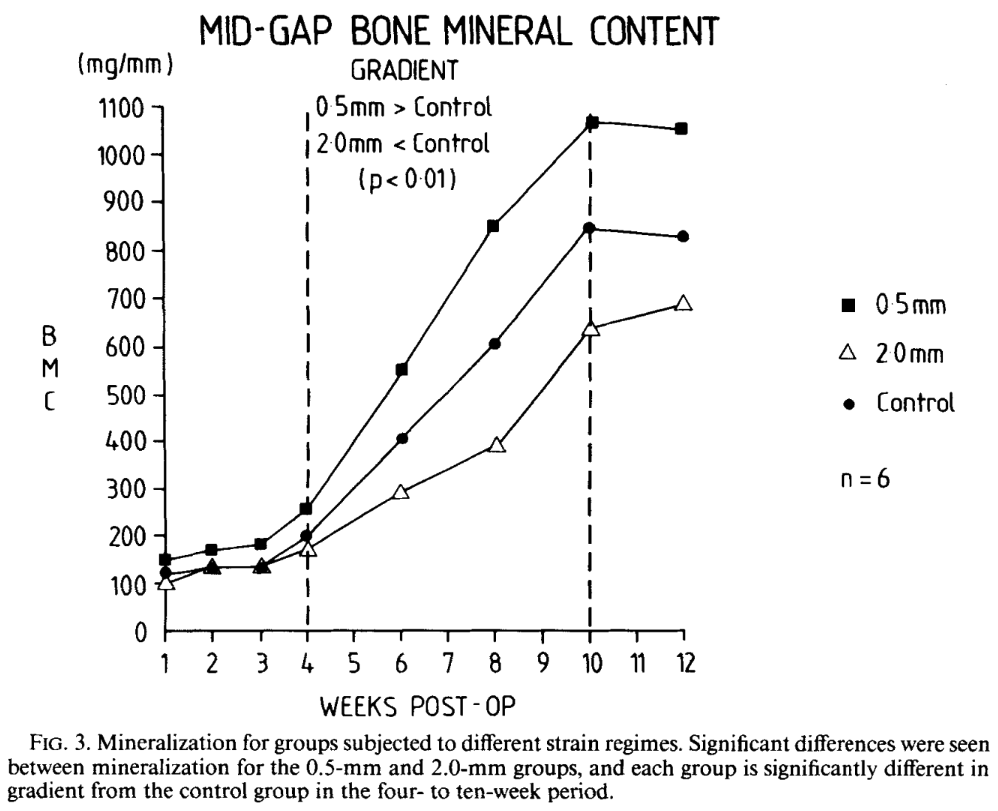
Magnitudes of local stress & strain along bony surfaces
Predict the course & type of fracture healing!
Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing
L.E. Claes, C.A. Heigele, (1999)
Magnitudes of local stress & strain along bony surfaces
Predict the course & type of fracture healing!
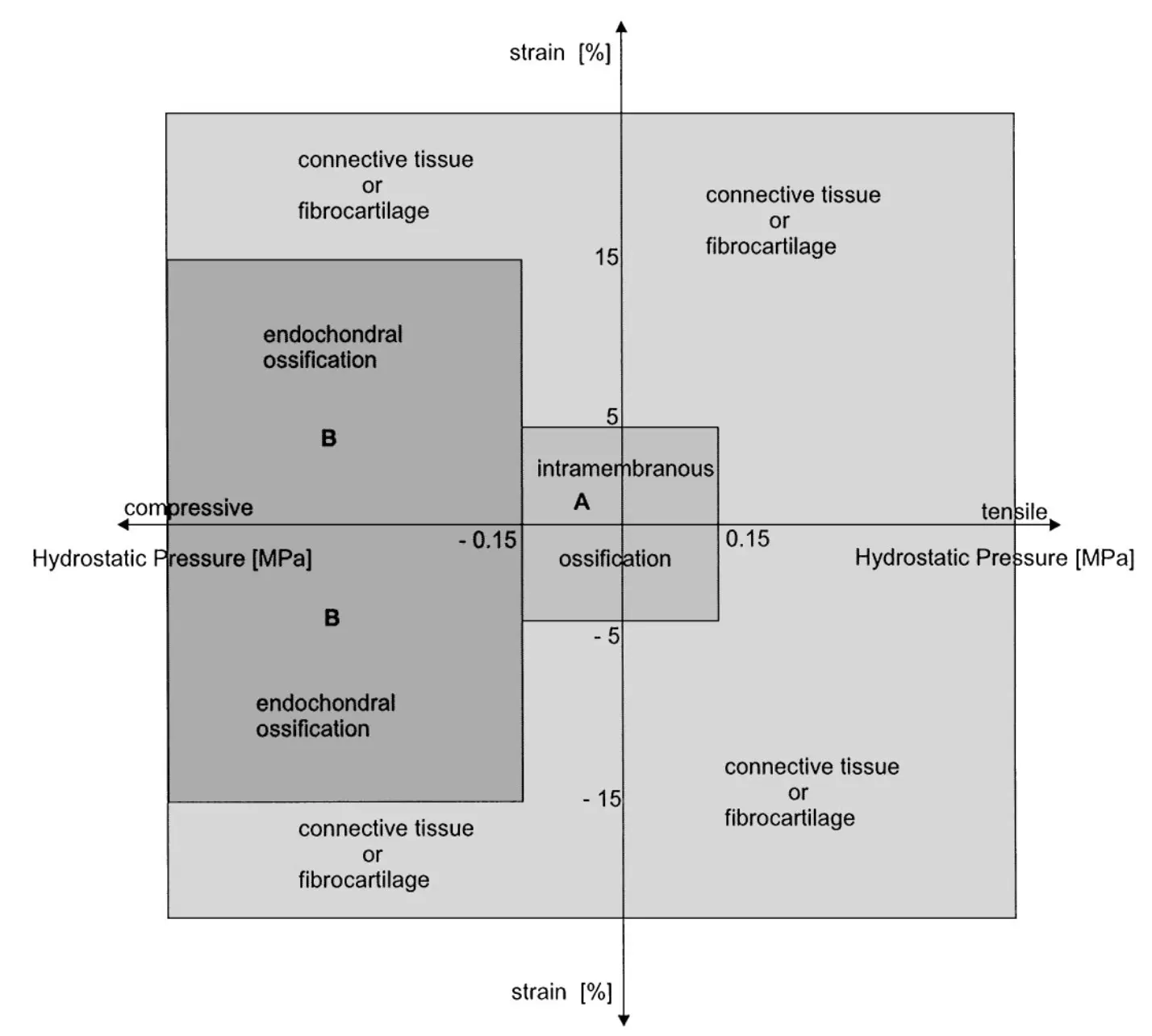
Dependence of healing on mechanical conditions:
Strain <5% & pressure <0.15 MPa → intramembranous bone formation.
Pressure ≈0.15 MPa → endochondral ossification.
High strain and pressure → fibrous tissue or cartilage.
Optimal interfragmentary mobility:
Initial mobility of ~1.2 mm stimulates callus formation. (IFM)
Gradual reduction of IFM due to increasing callus stiffness.
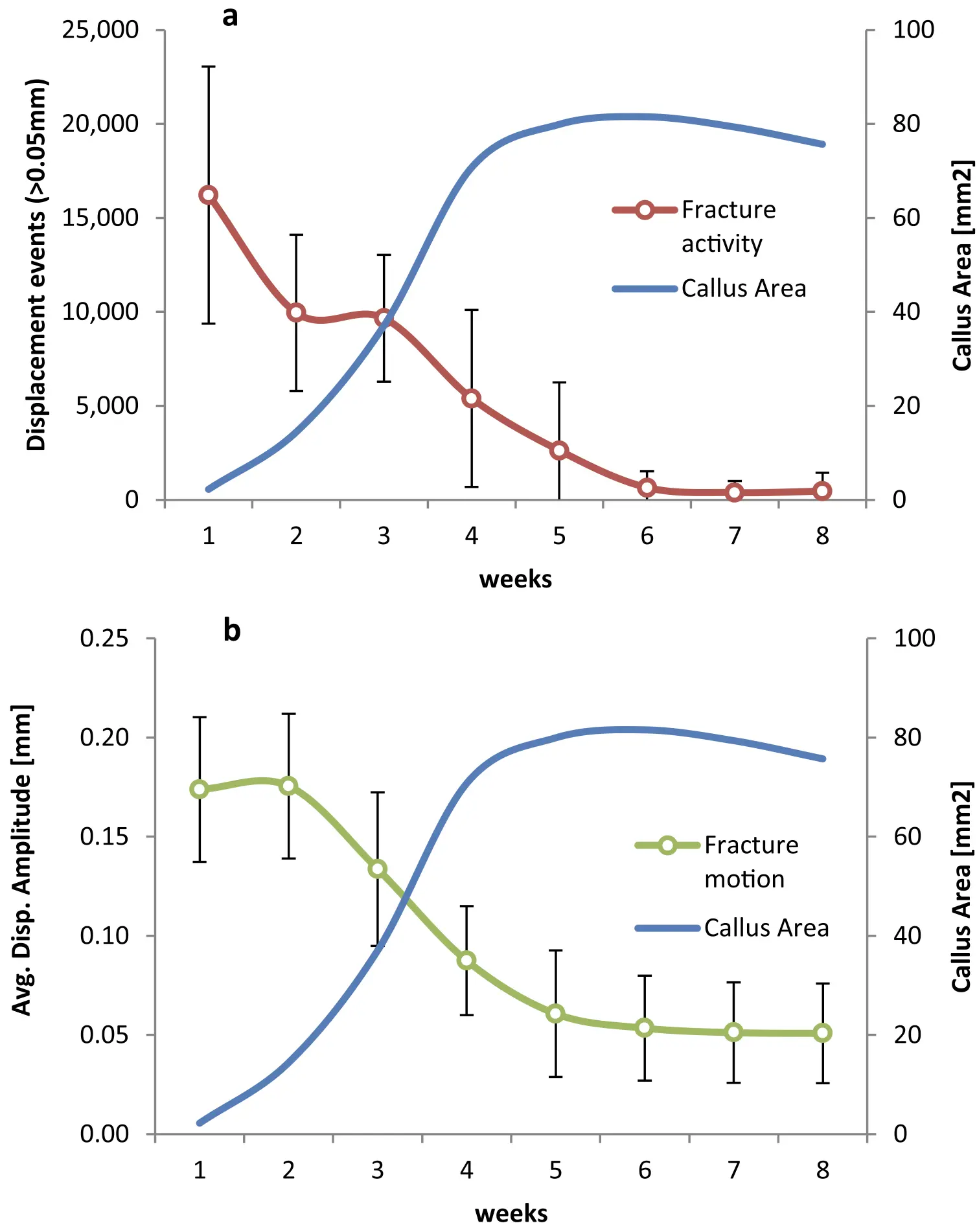
Reverse Dynamisation Boosts Healing by Controlling Strain
Reverse Dynamisation: A Modern Perspective On Stephan Perren’s Strain Theory
V. Glatt, C.H. Evans and K. Tetsworth, (2021)
Reverse Dynamisation Boosts Healing by Controlling Strain
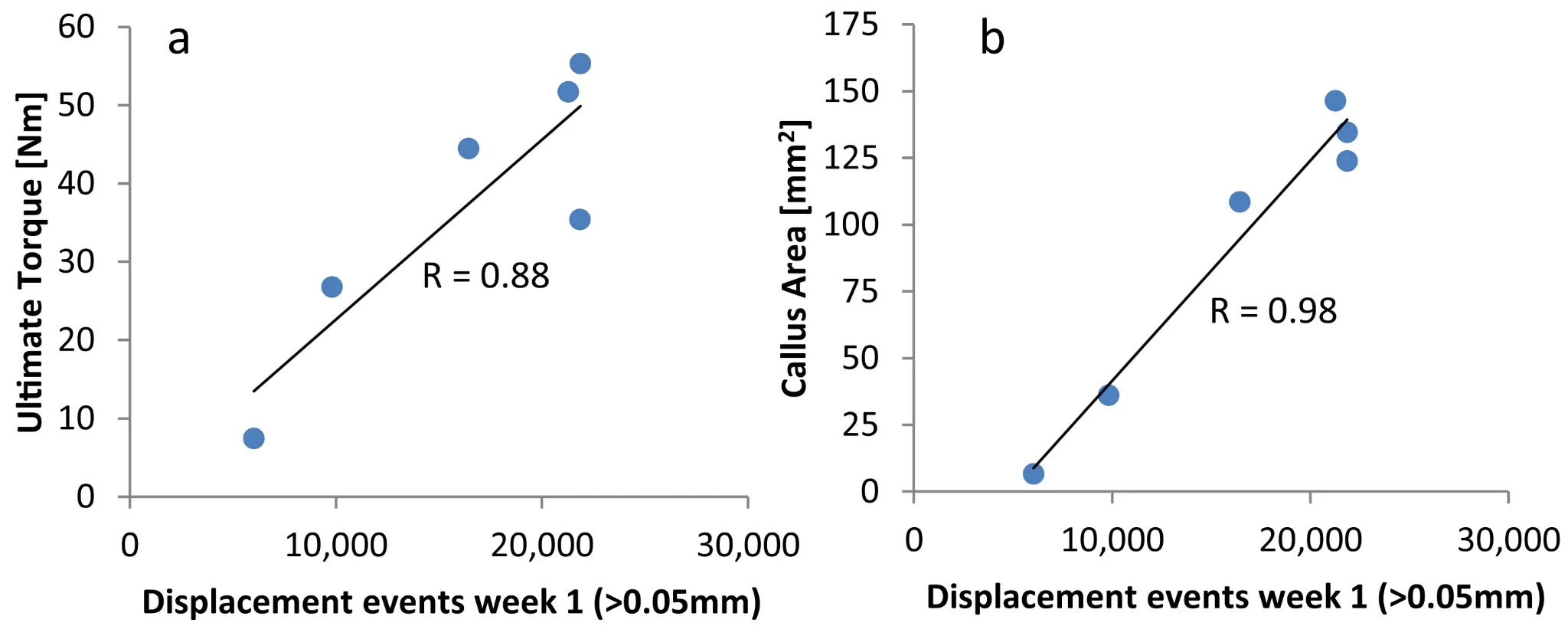
Early Fracture Activity is Crucial for Bone Regeneration
The relation between fracture activity and bone healing with special reference to the early healing phase – A preclinical study
Markus Windolf, Manuela Ernst, Ronald Schwyn, Daniel Arens, Stephan Zeiter, (2021)
Early Fracture Activity is Crucial for Bone Regeneration
Validation Testing of a New Crutch Tip Biofeedback Device for Prescribed Lower Extremity Weight-Bearing
Kevin E. Brueilly, Amanda M. Feller, Jonathan M. Ahearn, Jonathan S. Goodwin (January 2024.)
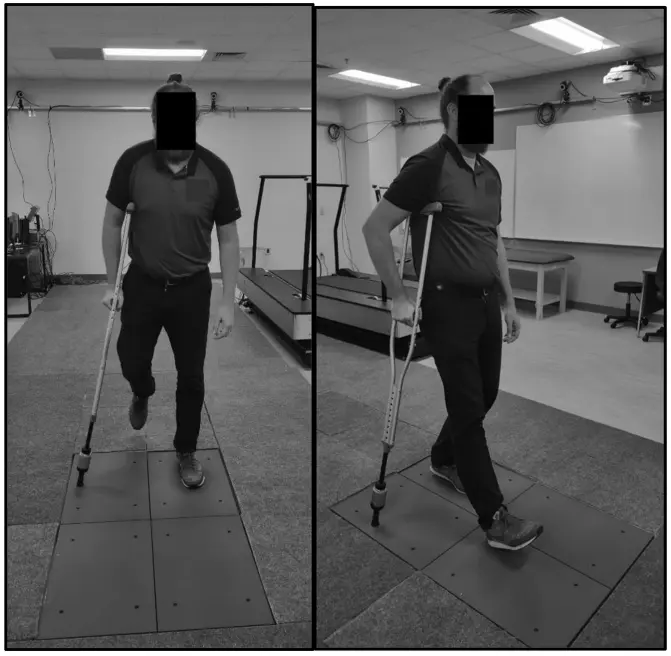

The ComeBack Mobility crutch tip system could be useful and should be considered for clinical use as a reliable and valid tool in providing auditory feedback for compliance to a prescribed weight-bearing protocol.
ComeBack Mobility Crutch Tip System
Improves patients weight-bearing compliance and satisfaction!
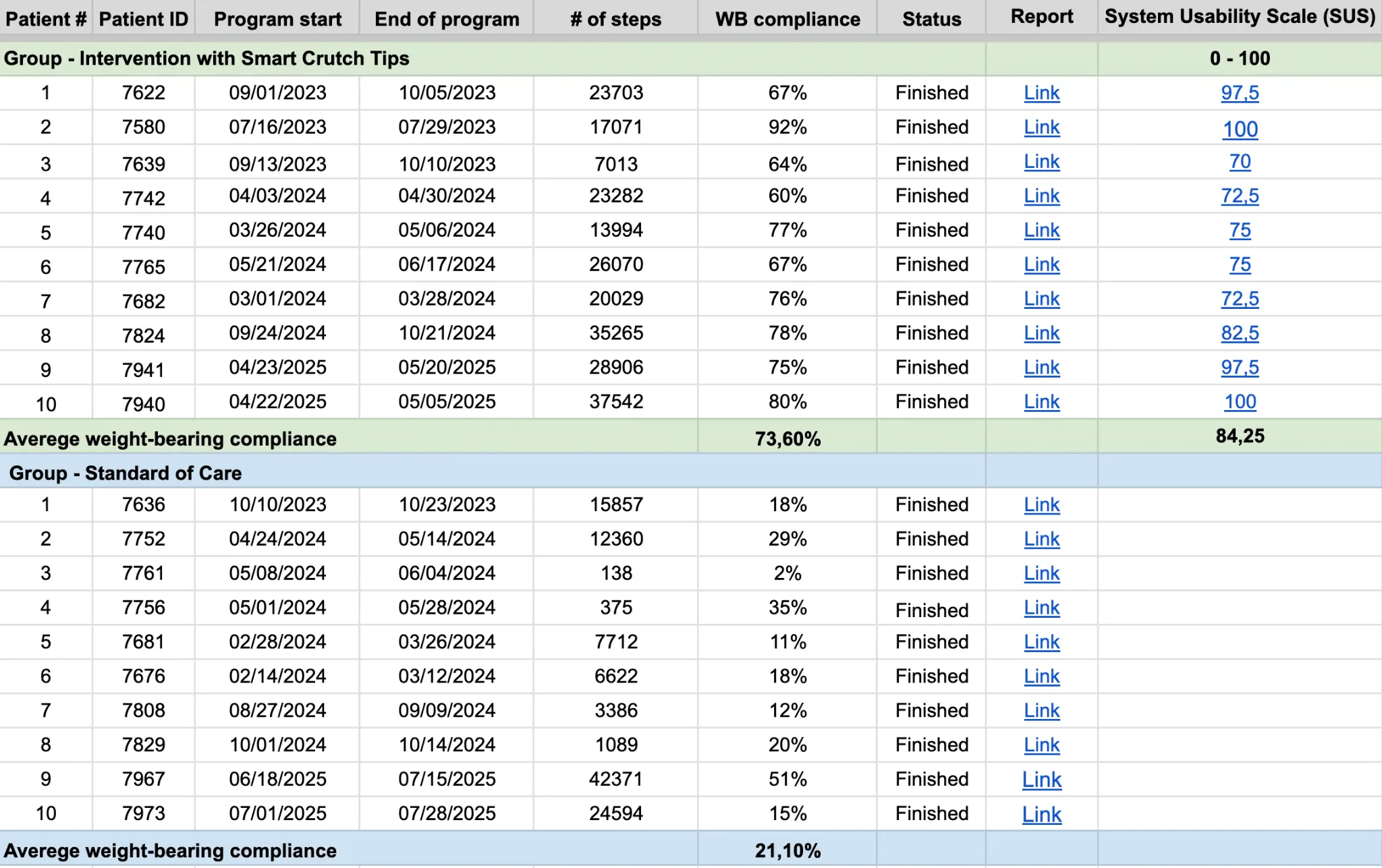
Smart Crutch Tips Enhance Weight-Bearing Adherence and Usability in Home-Based Rehabilitation (July 2025)
ComeBack Mobility Crutch Tip System
Improves patients weight-bearing compliance and satisfaction!
Weight-bearing compliance:
73.60% in Intervention group vs. 21.10% in Control group.
Usability:
SUS score of 84.25 – “Excellent Usability.” Patient Satisfaction: 10/10 likelihood to recommend the device.
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Tibial Shaft Fractures
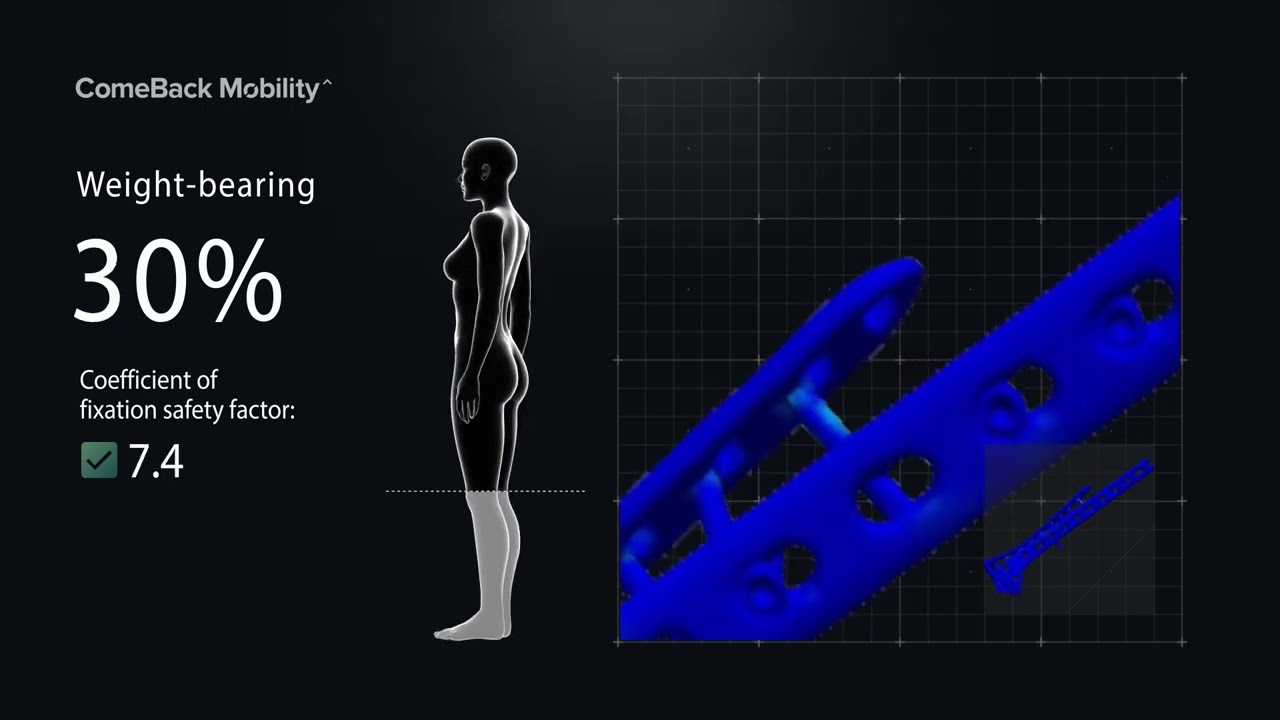
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Proximal Tibia Fractures
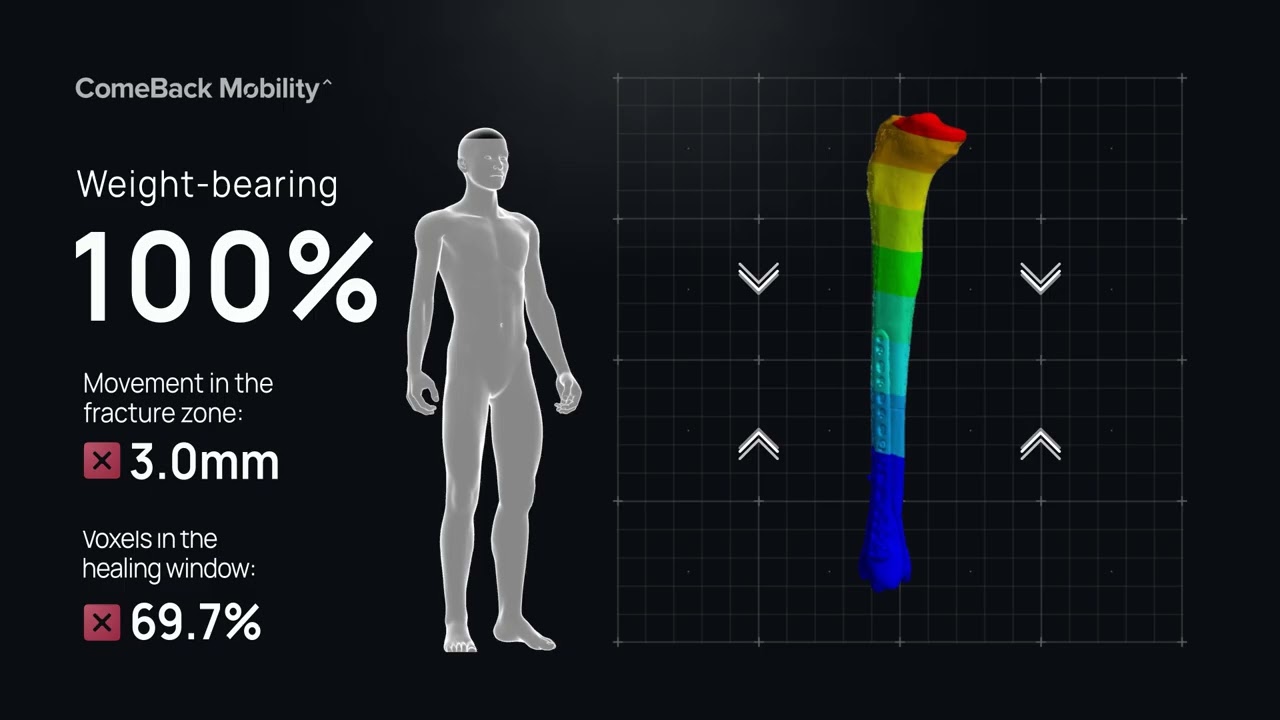
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Distal Tibia Fractures

With Smart Crutch Tips, your doctor can monitor the course of rehabilitation and help you avoid complications
a) loosening of osseous retainer screws
b) migration of screws or spokes
c) loosening of intramedullary retainer locking screws
d) loosening of the intramedullary shaft
e) loosening of the blade of the osseous plate or blocked epiphyseal screws (LCP, DHS, DCS systems)
f) teething of wire seam
a) deformation of the plate
b) deformation of the intramedullary shaft
c) deformation of the locking screws of the intramedullary retainer
a) loosening or teething of spokes or transosseous rods of an external fixer
b) fracture of spokes or transosseous rods of an external fixator
c) destabilization or damage to the external structure of the AVF
a) transplant migration
b) transplant fracture
c) fixation migration after consolidation is completed
a) vein thrombosis of the lower extremities
b) thromboembolic complications
c) muscle and joint contractures
d) muscle weakness and muscle volume reduction
e) gait stereotype disturbances
a) fixation plates and screws break muscle weakness
b) Dislocation of prosthesis joint contractures
c) Bone density loss gait disturbances
d) Blood clots
e) Muscle atrophy
Orthopedic Trauma Surgery
Chief of Trauma Division in NYU Langone Health
23+ Yrs Experience
“It gives them immediate feedback and teaches them had to weight bear properly and follow up the follows a program that I prescribed gives me feedback”
CSU Prof. & Assoc. Director Physical Therapy
![]()
“It gives everybody an opportunity to just have some more feedback”
Maastricht University Medical Center, the Netherlands. Does research in Surgery and Traumatology. Current project – ‘Permissive weight bearing’
“With the feedback patients gets from the Crutches, they will back to walk 8 weeks sooner”
Doctor of Physical Therapy, Regional Director
Moriarty Physical Therapy
![]()
“The biggest issue for me is that people aren’t listening, so it’s an issue of not enough pressure or too much pressure. With teenagers it’s a little bit less of «too much», it’s a matter of putting enough weight to it, so I can track it. I can see their percent, so when they come I can say: «Hey, you are not doing enough. You’ve make a thousand steps the first week and week 2 you kinda fall off. You have to stop your game up and get more compliance to put more pressure or ask them not to put too much pressure”
PT, DPT, CSCS, USAW, SFMA, TPI, Clinical Director Professional Care Physical Therapy
![]()
“The issue arise is that one the patient foot is out of the scale, really there is no other way to tell how much weight they are actually putting. There is no objective medical founded”
With Smart Crutch Tips, your doctor can monitor the course of rehabilitation and help you avoid complications
a) loosening of osseous retainer screws
b) migration of screws or spokes
c) loosening of intramedullary retainer locking screws
d) loosening of the intramedullary shaft
e) loosening of the blade of the osseous plate or blocked epiphyseal screws (LCP, DHS, DCS systems)
f) teething of wire seam
a) deformation of the plate
b) deformation of the intramedullary shaft
c) deformation of the locking screws of the intramedullary retainer
a) loosening or teething of spokes or transosseous rods of an external fixer
b) fracture of spokes or transosseous rods of an external fixator
c) destabilization or damage to the external structure of the AVF
a) fixation plates and screws break muscle weakness
b) Dislocation of prosthesis joint contractures
c) Bone density loss gait disturbances
d) Blood clots
e) Muscle atrophy
a) transplant migration
b) transplant fracture
c) fixation migration after consolidation is completed
a) vein thrombosis of the lower extremities
b) thromboembolic complications
c) muscle and joint contractures
d) muscle weakness and muscle volume reduction
e) gait stereotype disturbances
Regarding the physicians using our product, we have been working with orthopedic surgeons and rehabilitation specialists in several leading healthcare institutions.
The idea of attaching Smart Tips to crutches was tested with real patients, and unlike insoles, Smart Crutch Tips are:
– Always with the patient, even at night, when the patient is barefoot
– More durable – 3 years of use
– Available to consumers of any age and shoe size
– More affordable to implement
– Fit the reusable model
When walking on crutches, there is a moment during which the healthy leg is completed lifted off the ground and the entire load is distributed between the crutches and the injured leg.
We can determine how much load is placed on the injured limb by subtracting the amount of weight on the crutches from the patient’s body weight. For example: if a patient’s weight is 80 kg and during a step he transferred 60 kg to crutches, then 20kg of pressure was exerted on the injured limb.
The accuracy of Smart Crutch Tips is 98,5%.
The amount of initial weight bearing can be set from 0% NWB to 50% PWB. The upper threshold for graduated WBAT is 80%.
The Smart Crutch Tips device can be used by patients recovering from nonsurgical and surgical treatments for hip, thigh, knee, shin, ankle, and foot injuries and pathologies
Yes, Canes with diameters from 17 to 30mm. A patients can begin their gait rehabilitation on crutches and switch to a cane for quality gait progression.
No, it doesn’t need FDA approval. It’s Medical Device class II, 501 (k) Exempt. It’s FDA registered and has all necessary regulatory approvals for official sales in the US market.
Yes, it’s covered by insurance. The device usage itself doesn’t cover due to new technology on the market. However, the doctors work is covered. So they can get additional money for device setup and biofeedback patient training and Remote Patient Monitoring (RPM).
– Yes. We change the devices if anything happens during patient usage.
– Warranty for hospitals – 1 year.
– However, we can provide an expanded warranty for hospitals for up to 3 years.
Yes, it has protection from dust and water – IP 54. It can be used while rain or snow and operates in temperatures: from 5F to 86F.
Weight-bearing tracking service to control the load on the injured leg during rehabilitation
ComeBack Mobility™ FDA Registration Number: 10083584 All rights reserved 2020-2026 Terms of Use and Privacy Policy
Specification Developer Office:
700 N St Mary's Street, Floor 14, Office 65,
San Antonio, TX, 78205, US
Contact us:
popov@comebackmobility.com
9 am - 6 pm CT
Contract Manufacture Office:
Batumska street 11, Office 211,
Dnipro, 49074, Ukraine
For inquiries regarding collaboration on
clinical study in Ukraine, contact us at:
+380-(98)-336-37-03
help@comebackmobility.com