Help patients adhere weight-bearing protocols to enhance bone healing & prevent fixation failure. These protocols reduce complications that lead to reoperations, generating additional revenue for practices and savings for payers.
Help patients adhere weight-bearing protocols to enhance bone healing & prevent fixation failure. These protocols reduce complications that lead to reoperations, generating additional revenue for practices and savings for payers.

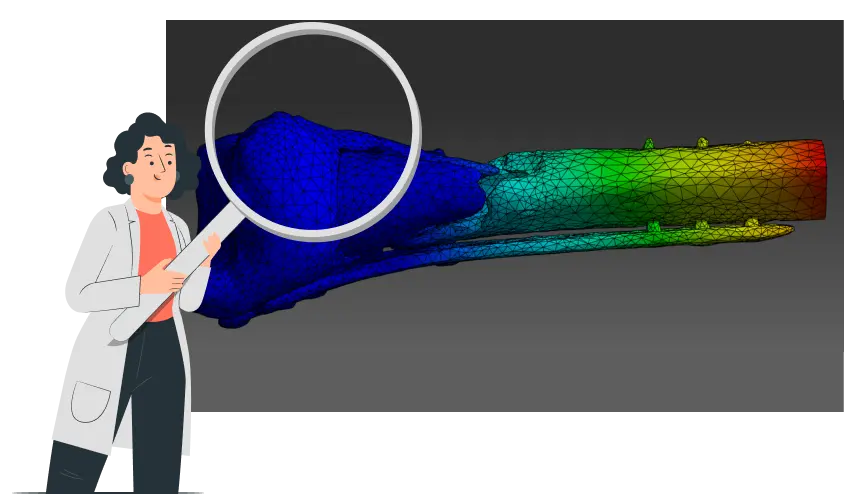
1. *CBM create 3D model from a post-op CT scan & calculate what load help the bone heal without compromising fixation
2. via *CBM app prescribe personalized load for the patient & remotely monitors compliance
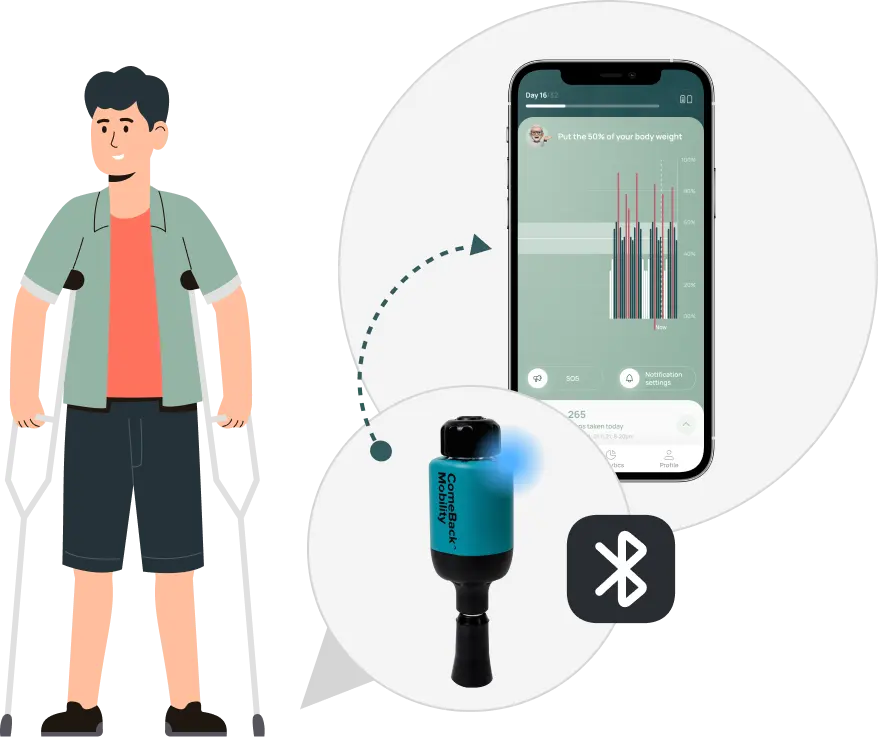
3. Patient use *CBM app & FDA cleared Crutch Tips to follow the prescribed loading plan.

1. *CBM create 3D model from a post-op CT scan & calculate what load help the bone heal without compromising fixation

2. via *CBM app prescribe personalized load for the patient & remotely monitors compliance

3. Patient use *CBM app & FDA cleared Crutch Tips to follow the prescribed loading plan.

ComeBack Mobility provides personalized post-operative rehabilitation protocols for lower-limb injuries, delivered through Smart Crutch Tips™ – an FDA-cleared device that helps patients accurately follow prescribed weight-bearing and step targets. These protocols are generated from CT-based finite-element analysis, allowing for the tailoring of loading and activity levels to achieve optimal interfragmentary motion, relevant strain at the fracture site, and fixation safety throughout healing.
Traditional rehabilitation lacks individualized biomechanical guidance. Surgeons rely on subjective judgments of fixation stability and often prescribe 6–12 weeks of no weight-bearing, which can lead either to excessive loading later causing implant failure or fracture displacement, or to prolonged immobilization, increasing the risks of deep vein thrombosis and muscular atrophy.
By enabling safe, earlier mobilization, we reduce complications that prolong recovery and drive costs. Hospitals benefit through shorter length of stay and fewer readmissions. For payers, preventing these events lowers the need for costly revision surgeries and reduces long-term disability expenses. Faster recovery helps patients return to work sooner, improving both clinical and economic outcomes.
in their rehabilitation
axial load
step data
of pain and swelling
Weight-bearing as tolerated is not always safe!
Individualized Determination of the Mechanical Fracture Environment After Tibial Exchange Nailing—A Simulation-Based Feasibility Study
Benedikt J. Braun, Marcel Orth, Stefan Diebels, Kerstin Wickert, Annchristin Andres, Joshua Gawlitza, Arno Bücker, Tim Pohlemann, Michael Roland (2021)
Weight-bearing as tolerated is not always safe!
Case:
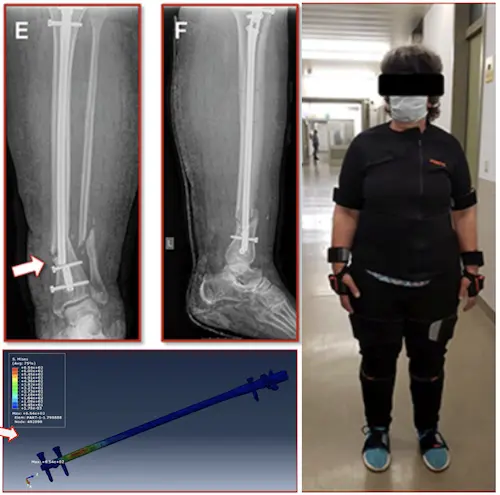
A 55-year-old woman broke a leg, 7 weeks after surgery, the metal piece used to fix her bone broke, and her leg broke again
Why It Happen?
Researchers found that too much load during walking caused the implant to fail
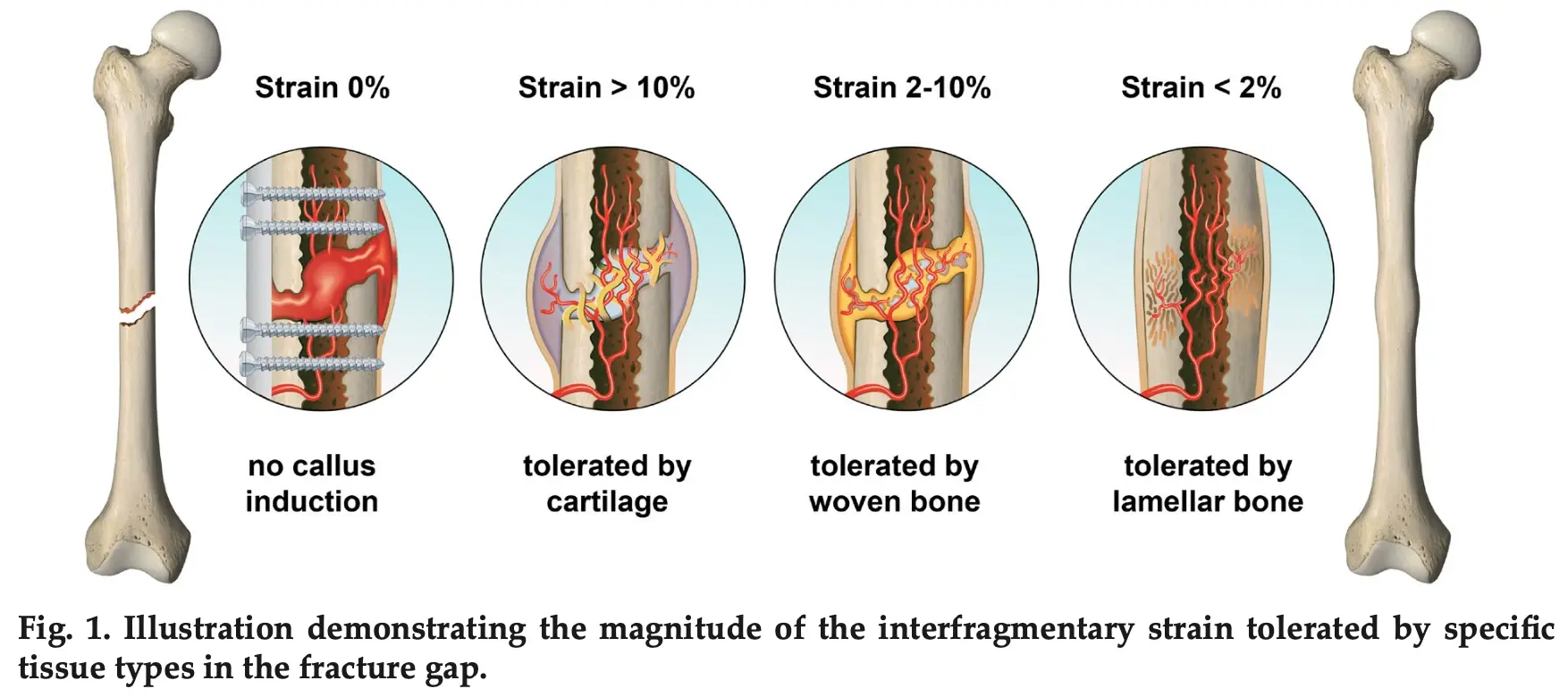
Applying Right Amount Of Strain Improves Healing!
If strain goes beyond certain limits – it slows down the healing process!
Controlled Mechanical Stimulation in the Treatment of Tibial Fractures
John Kenwright, Ph.D., F.R.C.S., And Allen E. Goodship,H.D., D.V.Sc., M.R.C.V.S., (1988)
Applying Right Amount Of Strain Improves Healing!
If strain goes beyond certain limits – it slows down the healing process!
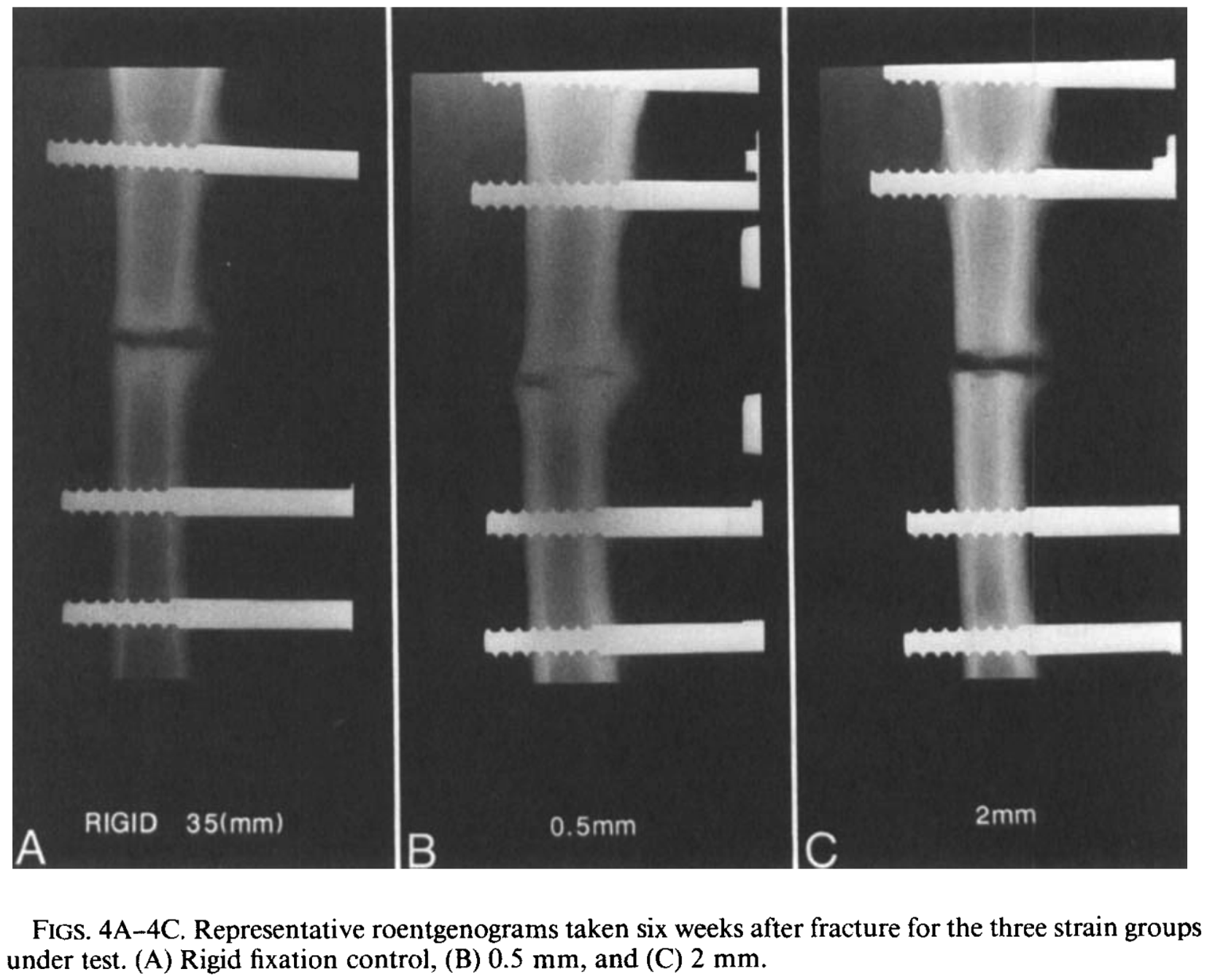
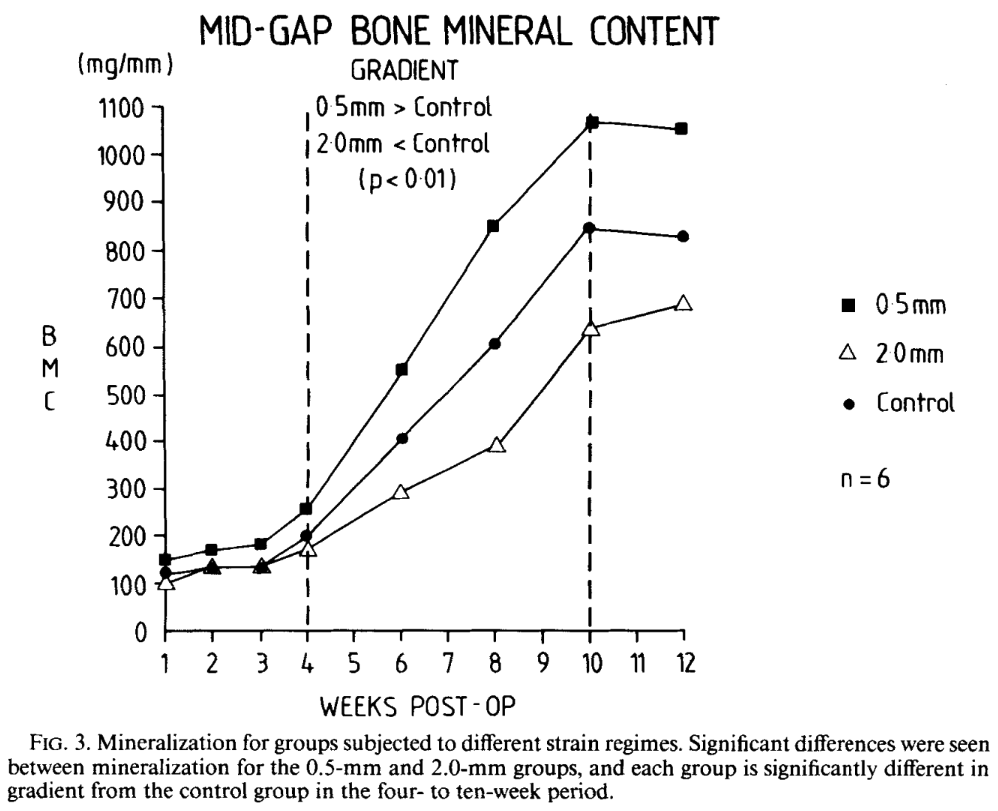
Magnitudes of local stress & strain along bony surfaces
Predict the course & type of fracture healing!
Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing
L.E. Claes, C.A. Heigele, (1999)
Magnitudes of local stress & strain along bony surfaces
Predict the course & type of fracture healing!
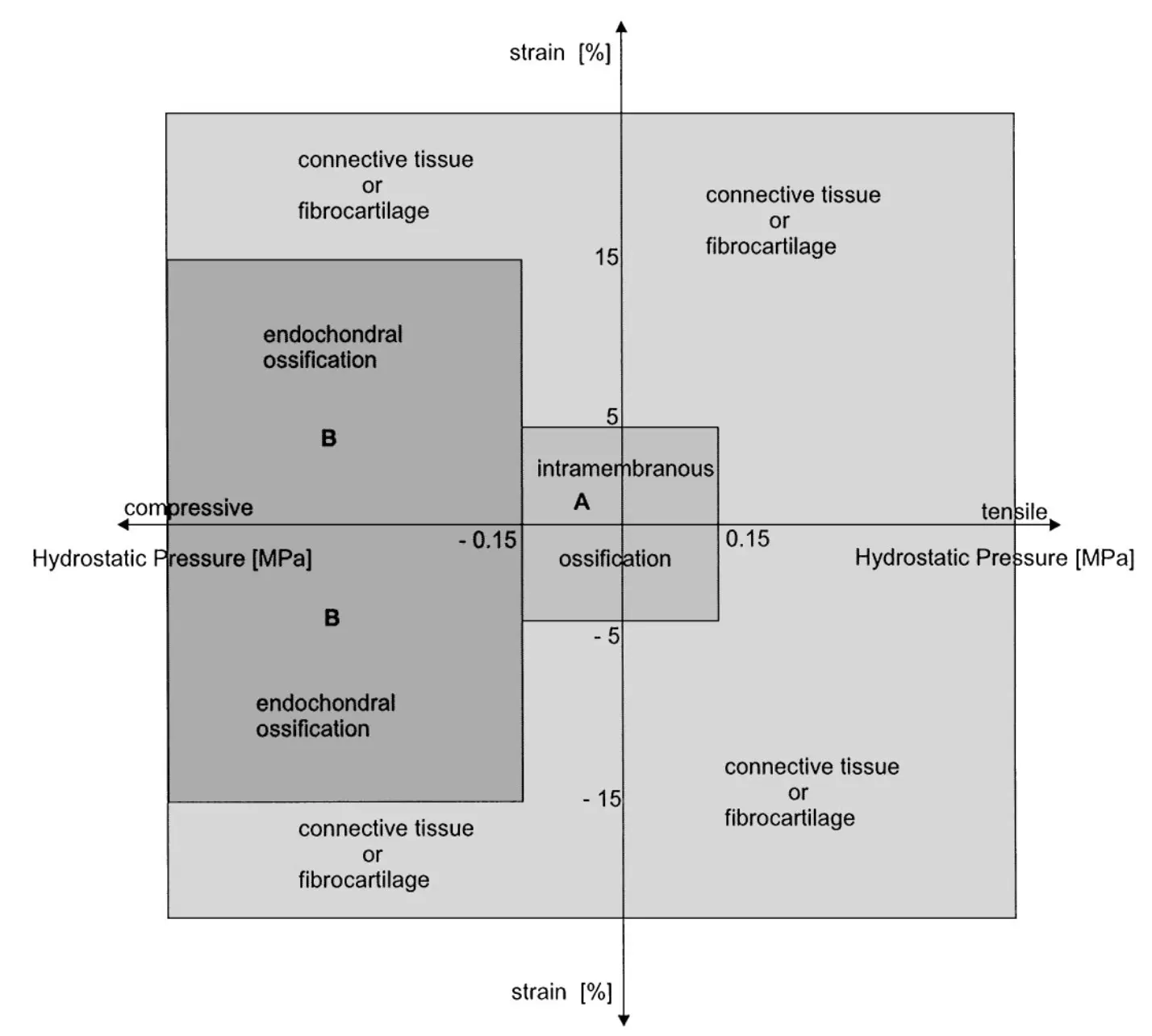
Dependence of healing on mechanical conditions:
Strain <5% & pressure <0.15 MPa → intramembranous bone formation.
Pressure ≈0.15 MPa → endochondral ossification.
High strain and pressure → fibrous tissue or cartilage.
Optimal interfragmentary mobility:
Initial mobility of ~1.2 mm stimulates callus formation. (IFM)
Gradual reduction of IFM due to increasing callus stiffness.
Reverse Dynamisation Boosts Healing by Controlling Strain
Reverse Dynamisation: A Modern Perspective On Stephan Perren’s Strain Theory
V. Glatt, C.H. Evans and K. Tetsworth, (2021)
Reverse Dynamisation Boosts Healing by Controlling Strain
Early Fracture Activity is Crucial for Bone Regeneration
The relation between fracture activity and bone healing with special reference to the early healing phase – A preclinical study
Markus Windolf, Manuela Ernst, Ronald Schwyn, Daniel Arens, Stephan Zeiter, (2021)
Early Fracture Activity is Crucial for Bone Regeneration
Validation Testing of a New Crutch Tip Biofeedback Device for Prescribed Lower Extremity Weight-Bearing
Kevin E. Brueilly, Amanda M. Feller, Jonathan M. Ahearn, Jonathan S. Goodwin (January 2024.)

The ComeBack Mobility crutch tip system could be useful and should be considered for clinical use as a reliable and valid tool in providing auditory feedback for compliance to a prescribed weight-bearing protocol.
ComeBack Mobility Crutch Tip System
Improves patients weight-bearing compliance and satisfaction!
Smart Crutch Tips Enhance Weight-Bearing Adherence and Usability in Home-Based Rehabilitation (July 2025)
ComeBack Mobility Crutch Tip System
Improves patients weight-bearing compliance and satisfaction!
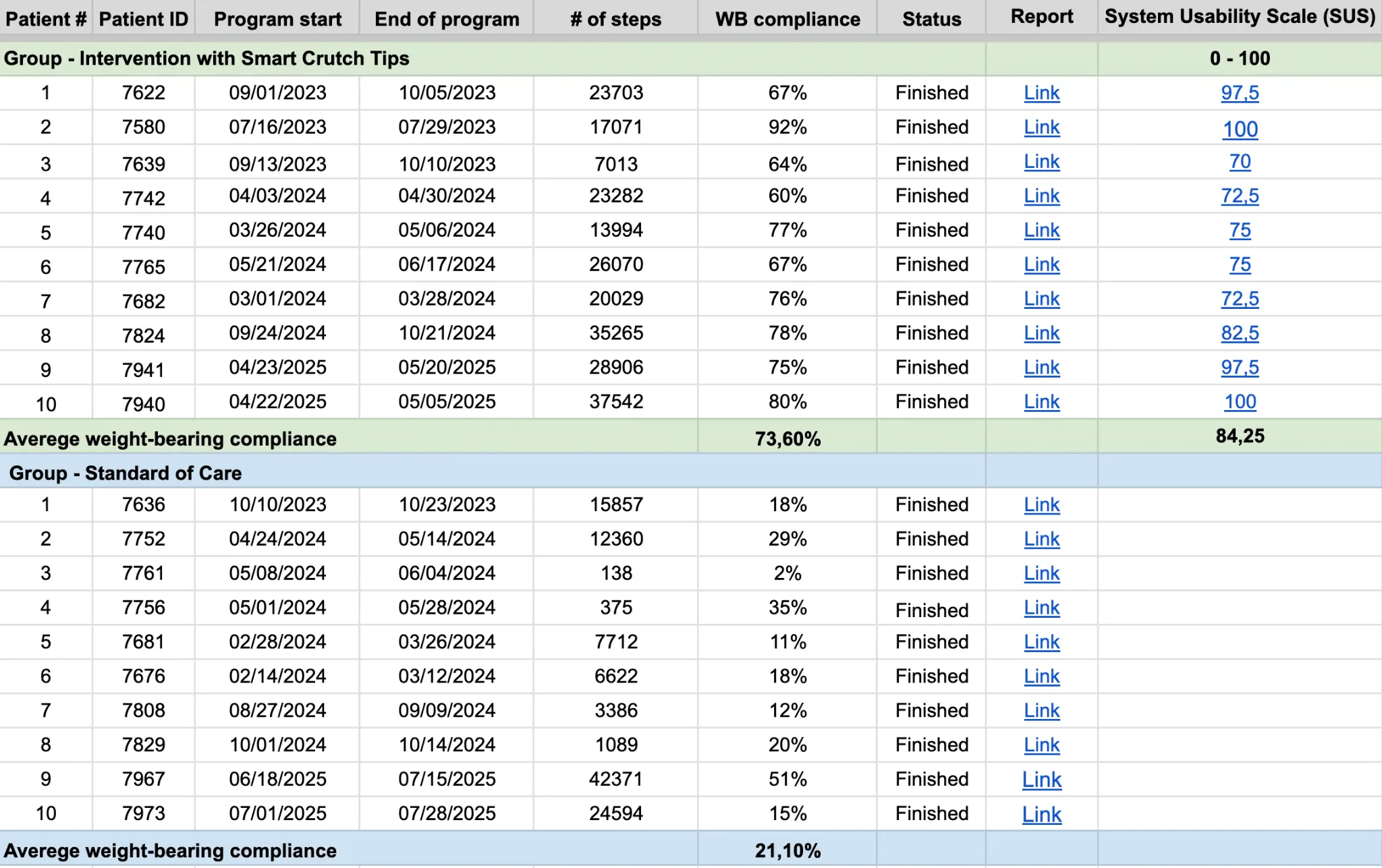
Weight-bearing compliance:
73.60% in Intervention group vs. 21.10% in Control group.
Usability:
SUS score of 84.25 – “Excellent Usability.” Patient Satisfaction: 10/10 likelihood to recommend the device.
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Tibial Shaft Fractures
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Proximal Tibia Fractures
Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Distal Tibia Fractures
Validation Testing of a New Crutch Tip Biofeedback Device for Prescribed Lower Extremity Weight-Bearing
Brueilly et al.

“Smart Crutch” Real Time Feedback Aids in Partial Weight-Bearing Adherence Following Lower Extremity Fracture: A Pilot Study
Kenneth A. Egol, MD et al.

Smart Crutch Tips for Guided Weight-Bearing
in Patients Recovering From Tibial Shaft
Fractures ClinicalTrials.gov ID NCT07092579
Glatt et al.

Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Proximal Tibia Fractures ClinicalTrials.gov ID NCT07134257
Glatt et al.

Smart Crutch Tips for Guided Weight-Bearing in Patients Recovering From Extra-Articular Distal Tibia Fractures ClinicalTrials.gov ID NCT07138066
Glatt et al.

Controlled Mechanical Stimulation in the Treatment of Tibial Fractures
Kenwright et al.

The relation between fracture activity and bone healing with special reference to the early healing phase – A preclinical study
Windolf et al.

Reverse Dynamisation: A Modern Perspective On Stephan Perren’s Strain Theory
Glatt et al.

Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing
Kenwright et al.

Individualized Determination of the Mechanical Fracture Environment After Tibial Exchange Nailing—A Simulation-Based Feasibility Study
Benedikt et al.

| Tear pattern | |||||
| Longitudinal-vertical (bucket handle tear) | Horizontal | Radial | Vertical flap | Horizontal flap | Complex |
| Tear depth | |||||
| Partial/ complete | |||||
| Radial location | |||||
| Posterior/Mid body/Anterior | |||||
| Location – width | |||||
| Zone 1 – Red-Red / Zone 2 – White-Red / Zone 3 – White-White | |||||
| Quality of tissue | |||||
| Non-degenerative / Degenerative / Undetermined | |||||
ACL/PCL
| ||||
MCL/LCL |
Type 1 / Type 2 / Type 3 / Type 4
| Internal fixation | External fixation |
| Precise / Fitbone / ISKD | Ilizarov / TSF (Taylor Spatial Frame) / Monolateral Fixator |
| Total / Partial |
| Revision / Re-revision |
| Total / Partial |
| Revision / Re-revision |
With Smart Crutch Tips, your doctor can monitor the course of rehabilitation and help you avoid complications
a) loosening of osseous retainer screws
b) migration of screws or spokes
c) loosening of intramedullary retainer locking screws
d) loosening of the intramedullary shaft
e) loosening of the blade of the osseous plate or blocked epiphyseal screws (LCP, DHS, DCS systems)
f) teething of wire seam
a) deformation of the plate
b) deformation of the intramedullary shaft
c) deformation of the locking screws of the intramedullary retainer
a) loosening or teething of spokes or transosseous rods of an external fixer
b) fracture of spokes or transosseous rods of an external fixator
c) destabilization or damage to the external structure of the AVF
a) transplant migration
b) transplant fracture
c) fixation migration after consolidation is completed
a) vein thrombosis of the lower extremities
b) thromboembolic complications
c) muscle and joint contractures
d) muscle weakness and muscle volume reduction
e) gait stereotype disturbances
a) fixation plates and screws break muscle weakness
b) Dislocation of prosthesis joint contractures
c) Bone density loss gait disturbances
d) Blood clots
e) Muscle atrophy
Orthopedic Trauma Surgery
Chief of Trauma Division in NYU Langone Health
23+ Yrs Experience
“It gives them immediate feedback and teaches them had to weight bear properly and follow up the follows a program that I prescribed gives me feedback”
CSU Prof. & Assoc. Director Physical Therapy

“It gives everybody an opportunity to just have some more feedback”
Maastricht University Medical Center, the Netherlands. Does research in Surgery and Traumatology. Current project – ‘Permissive weight bearing’
“With the feedback patients gets from the Crutches, they will back to walk 8 weeks sooner”
Doctor of Physical Therapy, Regional Director
Moriarty Physical Therapy

“The biggest issue for me is that people aren’t listening, so it’s an issue of not enough pressure or too much pressure. With teenagers it’s a little bit less of «too much», it’s a matter of putting enough weight to it, so I can track it. I can see their percent, so when they come I can say: «Hey, you are not doing enough. You’ve make a thousand steps the first week and week 2 you kinda fall off. You have to stop your game up and get more compliance to put more pressure or ask them not to put too much pressure”
PT, DPT, CSCS, USAW, SFMA, TPI, Clinical Director Professional Care Physical Therapy

“The issue arise is that one the patient foot is out of the scale, really there is no other way to tell how much weight they are actually putting. There is no objective medical founded”
With Smart Crutch Tips, your doctor can monitor the course of rehabilitation and help you avoid complications
a) loosening of osseous retainer screws
b) migration of screws or spokes
c) loosening of intramedullary retainer locking screws
d) loosening of the intramedullary shaft
e) loosening of the blade of the osseous plate or blocked epiphyseal screws (LCP, DHS, DCS systems)
f) teething of wire seam
a) deformation of the plate
b) deformation of the intramedullary shaft
c) deformation of the locking screws of the intramedullary retainer
a) loosening or teething of spokes or transosseous rods of an external fixer
b) fracture of spokes or transosseous rods of an external fixator
c) destabilization or damage to the external structure of the AVF
a) fixation plates and screws break muscle weakness
b) Dislocation of prosthesis joint contractures
c) Bone density loss gait disturbances
d) Blood clots
e) Muscle atrophy
a) transplant migration
b) transplant fracture
c) fixation migration after consolidation is completed
a) vein thrombosis of the lower extremities
b) thromboembolic complications
c) muscle and joint contractures
d) muscle weakness and muscle volume reduction
e) gait stereotype disturbances
Regarding the physicians using our product, we have been working with orthopedic surgeons and rehabilitation specialists in several leading healthcare institutions.
The idea of attaching Smart Tips to crutches was tested with real patients, and unlike insoles, Smart Crutch Tips are:
– Always with the patient, even at night, when the patient is barefoot
– More durable – 3 years of use
– Available to consumers of any age and shoe size
– More affordable to implement
– Fit the reusable model
When walking on crutches, there is a moment during which the healthy leg is completed lifted off the ground and the entire load is distributed between the crutches and the injured leg.
We can determine how much load is placed on the injured limb by subtracting the amount of weight on the crutches from the patient’s body weight. For example: if a patient’s weight is 80 kg and during a step he transferred 60 kg to crutches, then 20kg of pressure was exerted on the injured limb.
The accuracy of Smart Crutch Tips is 98,5%.
The amount of initial weight bearing can be set from 0% NWB to 50% PWB. The upper threshold for graduated WBAT is 80%.
The Smart Crutch Tips device can be used by patients recovering from nonsurgical and surgical treatments for hip, thigh, knee, shin, ankle, and foot injuries and pathologies
Yes, Canes with diameters from 17 to 30mm. A patients can begin their gait rehabilitation on crutches and switch to a cane for quality gait progression.
No, it doesn’t need FDA approval. It’s Medical Device class II, 501 (k) Exempt. It’s FDA registered and has all necessary regulatory approvals for official sales in the US market.
Yes, it’s covered by insurance. The device usage itself doesn’t cover due to new technology on the market. However, the doctors work is covered. So they can get additional money for device setup and biofeedback patient training and Remote Patient Monitoring (RPM).
– Yes. We change the devices if anything happens during patient usage.
– Warranty for hospitals – 1 year.
– However, we can provide an expanded warranty for hospitals for up to 3 years.
Yes, it has protection from dust and water – IP 54. It can be used while rain or snow and operates in temperatures: from 5F to 86F.
Weight-bearing tracking service to control the load on the injured leg during rehabilitation
ComeBack Mobility™ FDA Registration Number: 10083584 All rights reserved 2020-2026 Terms of Use and Privacy Policy
Specification Developer Office:
700 N St Mary's Street, Floor 14, Office 65,
San Antonio, TX, 78205, US
Contact us:
popov@comebackmobility.com
9 am - 6 pm CT
LLC "FISON." Contract Manufacture Office:
Batumska street 11, Office 211,
Dnipro, 49074, Ukraine
For inquiries regarding collaboration on
clinical study in Ukraine, contact us at:
+380-(98)-336-37-03
help@comebackmobility.com
Link to the ClinicalTrials.gov | NCT07138066
Brief Summary
The goal of this clinical trial is to learn whether personalized weight-bearing prescriptions using Smart Crutch Tips™ can improve recovery after surgery for extra-articular distal tibia fractures. The study will also assess how safe and practical this approach is in daily outpatient use.
Can a personalized weight-bearing program based on CT and finite element analysis help the fracture heal faster? Can it help patients return to full weight-bearing sooner? Can it reduce the fear of movement during recovery? Researchers will compare standard rehabilitation, AO Foundation-based recommendations, and personalized weight-bearing programs derived from finite element analysis (FEA) to determine which approach leads to faster healing, earlier mobility, and better outcomes.
Participants will: Use Smart Crutch Tips™ during walking for up to 24 weeks; Follow a personalized weight-bearing prescription based on CT scans and biomechanical modeling; Follow a specific walking plan with real-time audio and visual feedback; Attend eight follow-up visits over 36 weeks for clinical exams, x-rays, and CT scans; Complete online questionnaires about pain, activity, and fear of movement.
Link to the ClinicalTrials.gov | NCT07134257
Brief Summary
The goal of this clinical trial is to learn whether personalized weight-bearing prescriptions using Smart Crutch Tips™ can improve recovery after surgery for extra-articular proximal tibia fractures. The study will also assess how safe and practical this approach is in daily outpatient use.
Can a personalized weight-bearing program based on CT and finite element analysis help the fracture heal faster? Can it help patients return to full weight-bearing sooner? Can it reduce the fear of movement during recovery? Researchers will compare standard rehabilitation, AO Foundation-based recommendations, and personalized weight-bearing programs derived from finite element analysis (FEA) to determine which approach leads to faster healing, earlier mobility, and better outcomes.
Participants will: Use Smart Crutch Tips™ during walking for up to 24 weeks; Follow a personalized weight-bearing prescription based on CT scans and biomechanical modeling; Follow a specific walking plan with real-time audio and visual feedback; Attend eight follow-up visits over 36 weeks for clinical exams, x-rays, and CT scans; Complete online questionnaires about pain, activity, and fear of movement.
Link to the ClinicalTrials.gov | NCT07092579
Brief Summary
The goal of this clinical trial is to learn whether personalized weight-bearing prescriptions using Smart Crutch Tips™ can improve recovery after surgery for tibial shaft fractures. The study will also assess how safe and practical this approach is in daily outpatient use.
Can a personalized weight-bearing program based on CT and finite element analysis help the fracture heal faster? Can it help patients return to full weight-bearing sooner? Can it reduce the fear of movement during recovery? Does iterative walking in the early postoperative period support faster or better bone healing? Researchers will compare standard rehabilitation to different types of personalized weight-bearing programs to see which leads to faster healing, earlier mobility, and better outcomes.
Participants will: Use Smart Crutch Tips™ during walking for up to 24 weeks; Follow a personalized weight-bearing prescription based on CT scans and biomechanical modeling; Follow a specific walking plan with real-time audio and visual feedback; Attend six follow-up visits over 36 weeks for clinical exams, x-rays, and CT scans; Complete online questionnaires about pain, activity, and fear of movement.
https://drive.google.com/file/d/1wM1EgfgNMO-m2x0VqwrfPqUK_mgB0hE6/view?usp=sharing
Abstract
Purpose: To evaluate the effectiveness of immediate weight bearing feedback crutch tips, or so called “smart crutches” in assessing and promoting patient adherence to partial weight-bearing (PWB) protocols and to explore patient experiences with a device that provided real time feedback about weight bearing.
Methods: Twenty patients with lower extremity fractures who were allowed PWB utilizing real-time feedback crutch tips (RFC) were randomized into one of two groups: The feedback group used RFC (ComeBack Mobility, Kiev Ukraine), which provided real-time feedback on PWB compliance. The Control group also used RFC’s without real-time patient feedback. PWB compliance was defined as maintaining weight-bearing within ±10% of the prescribed range and was calculated as the percentage of steps meeting that threshold. Secondary outcomes included device usability and patient satisfaction, both assessed during the home rehabilitation period. Usability was measured via the System Usability Scale (SUS).
Results: The Intervention group demonstrated significantly higher PWB compliance than the Control group (73.60% vs. 21.10%, p < 0.01). The Intervention group reported an average SUS score of 84.25, indicating excellent usability. There were no complications or safety events related to the use of the devices. Patient satisfaction was evaluated through video interviews: patients interviewed in the Intervention group expressed high satisfaction and a strong willingness (10/10) to recommend the device for similar injuries.
Conclusion: RFCs were safe and effectively improved partial weight-bearing compliance during home rehabilitation. These findings support integrating such devices into wider clinical care to enhance patient outcomes and adherence to prescribed weight-bearing protocols.
Abstract
Introduction: Modified weight-bearing recommendations are commonly prescribed after surgical intervention for injuries to the lower extremity to reduce the risk of nonunion and delayed healing associated with load bearing through the injured limb and to combat the deleterious effects of immobility. The physical therapist (PT) in the acute care setting is likely to instruct patients after lower extremity injury in weight-bearing-restricted ambulation. A new device is now available for use in the training of weight-bearing status. The study examines whether the ComeBack Mobility crutch tip reporting weight-bearing on the lower extremity is a reliable and valid tool in determining force when compared with the gold standard force plate measurement of lower extremity weight-bearing.
Review of Literature: Previous studies have shown that patients are often not able to adequately learn or adhere to restrictive weight-bearing modifications. This may be due to an inability to provide immediate and ongoing feedback on weight-bearing. The new ComeBack Mobility crutch tip system is now available for the acute care PT to use in instruction and for patients to receive real-time feedback throughout their rehabilitation process.
Subjects: A sample of convenience of 6 able-bodied PTs was used.
Methods: Each subject performed 30 trials of axillary crutch-assisted weight-bearing ambulation using the new device. The weight-bearing reported by the device was compared with the weight-bearing measured through force plates via correlations, t tests, and Bland-Altman plot.
Results: The new device demonstrated moderate-good reliability in the measurement of non-weight-bearing and 50% partial weight-bearing in trials completed.
Discussion and Conclusion: The ComeBack Mobility crutch tip system could be useful and should be considered for clinical use as a reliable and valid tool in providing auditory feedback for compliance to a prescribed weight-bearing protocol. The system could be useful in the training of patients in the first use of crutches such as prior to discharge from an acute care hospital. Further research is needed with clinical populations as well as with varied weight-bearing protocols.
https://www.injuryjournal.com/article/S0020-1383(20)30851-2/abstract
Abstract
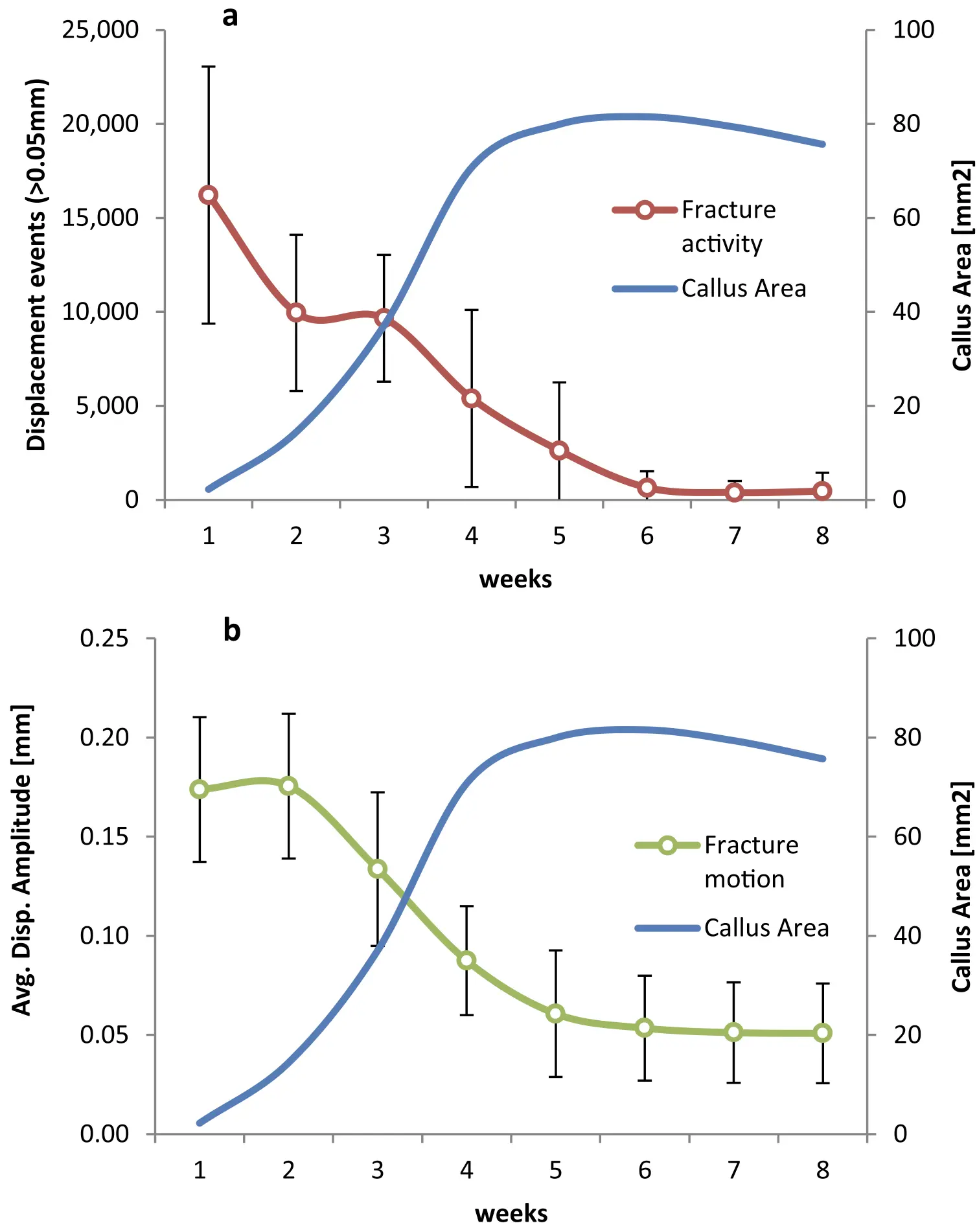
Background: Fracture healing outcome is to a great extent steered by the mechanical environment. The importance of early phase mechanical fracture stimulation is still controversially discussed, both clinically and scientifically. Furthermore, the role of fracture activity, defined as the number of stimulatory events per time, is particularly for the direct postoperative phase unknown.
Methods: Tibial defects of seven Swiss mountain sheep were stabilized with a dynamizable bone fixator, which allowed for defined interfragmentary motion by limiting the maximum axial displacement. The fixator was further equipped with a telemetric measuring unit to continuously log all occurring displacement events above a predefined amplitude threshold over an 8-weeks observation period. Callus size was measured over time from X-rays. Ultimate torsional strength of the healed defects was assessed after euthanasia.
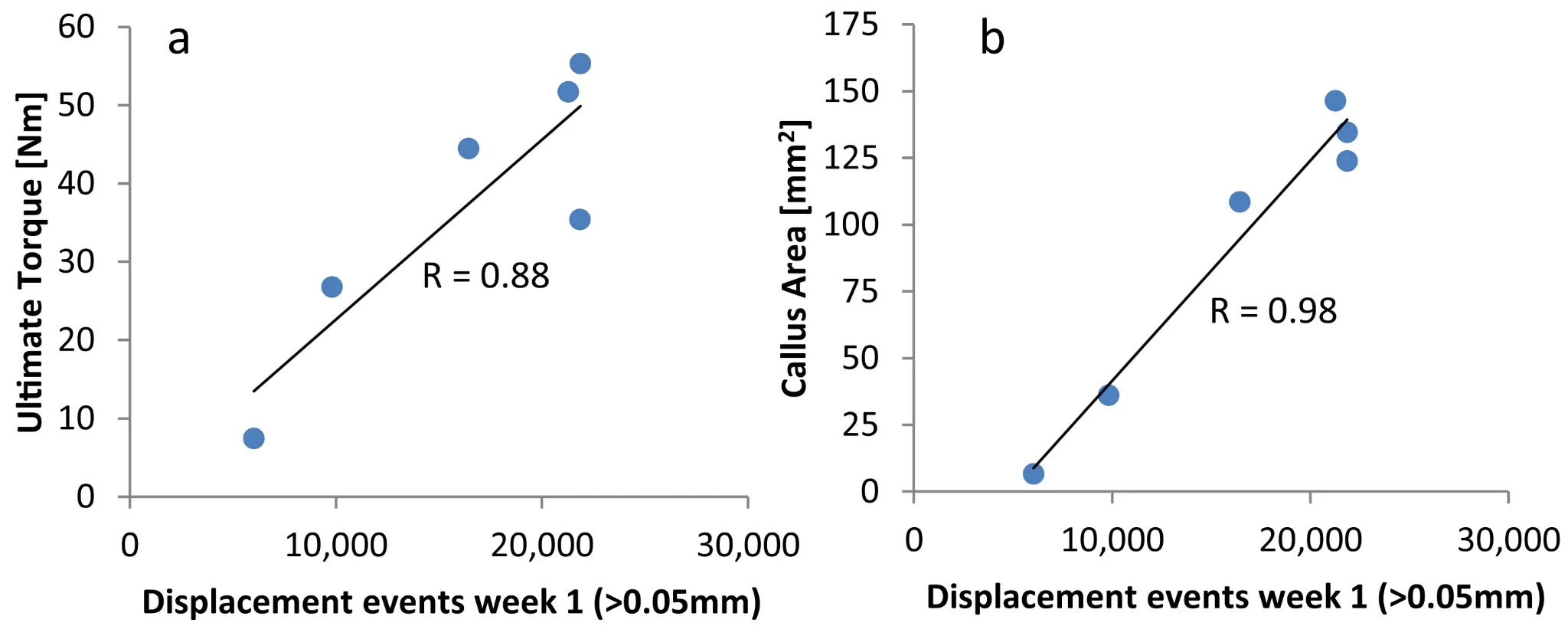
Results: One animal had to be excluded from the experiment due to technical reasons. The remaining six animals exhibited consistently the highest fracture activity in week 1 post-operation with 6′029 displacement events per week for the animal with the lowest activity and 21′866 events per week for the most active animal. Afterwards fracture activity gradually decreased over time. Strong and significant correlations were found for fracture activity in week 1 and 2 with torsional strength of the healed bone (R ≥ 0.881, p ≤ 0.02). No significant correlations were observed at later timepoints. Fracture activity in week 1 and 2 also correlated strongly with the maximum callus area as measured from X-rays (R ≥ 0.846, p ≤ 0.034).
Conclusions: The data demonstrates a positive effect of, within limits, frequent fracture stimulation on bone healing and suggests the importance of the mechanical environment in the direct post-operative healing phase. Clinically, the findings may advocate for the concept of direct post-operative weight bearing. This, however, requires clinical validation and must be considered within the full clinical context including the risk for fixation failure from overloading.
https://pubmed.ncbi.nlm.nih.gov/34111297/
Abstract
The present review acknowledges the tremendous impact of Stephan Perren’s strain theory, considered with respect to the earlier contributions of Roux and Pauwels. Then, it provides further insight by examining how the concept of reverse dynamisation extended Perren’s theory within a modern context. A key factor of this more contemporary theory is that it introduces variable mechanical conditions at different time points during bone healing, opening the possibility of manipulating biology through mechanics to achieve the desired clinical outcome. The discussion focusses on the current state of the art and the most recent advances made towards optimising and accelerating bone regeneration, by actively controlling the mechanical environment as healing progresses. Reverse dynamisation utilises a very specific mechanical manipulation regimen, with conditions initially flexible to encourage and expedite early callus formation. Once callus has formed, the mechanical conditions are intentionally modified to create a rigid environment under which the soft callus is quickly converted to hard callus, bridging the fracture site and leading to a more rapid union. The relevant literature, principally animal studies, was surveyed to provide ample evidence in support of the effectiveness of reverse dynamisation. By providing a modern perspective on Stephan Perren’s strain theory, reverse dynamisation perhaps holds the key to tipping the balance in favour of a more rapid and reliable union when treating acute fractures, osteotomies, non-unions and other circumstances where it is necessary to regenerate bone.
http://biomechanics.ru/lib/files/32_0255.pdf
Abstract
A new quantitative tissue differentiation theory which relates the local tissue formation in a fracture gap to the local stress and strain is presented. Our hypothesis proposes that the amounts of strain and hydrostatic pressure along existing calcified surfaces in the fracture callus determine the differentiation of the callus tissue. The study compares the local strains and stresses in the callus as calculated from a finite element model with histological findings from an animal fracture model. The hypothesis predicts intramembranous bone formation for strains smaller approximately 5% and hydrostatic pressures smaller than 0.15 MPa. Endochondral ossification is associated with compressive pressures larger than about –0.15 MPa and strains smaller than 15%. All other conditions seemed to lead to connective tissue or fibrous cartilage. The hypothesis enables a better understanding of the complex tissue differentiation seen in histological images and the mechanical conditions for healing, delayed healing, or nonunions. (1999 Elsevier Science Ltd. All rights reserved.)
https://drive.google.com/file/d/1sZ3h2vfcyjX3U9ZetaQfOz6vd_VCyAqr/view
Although it is known that the mechanical environment affects the fracture healing process, the optimal conditions for the different stages of healing have not been defined. In the present studies, the influence of applying a very short period of axial micromovement with defined characteristics to healing fractures has been studied both in simulated and clinical tibial fractures. The fracture healing process is seen to be acutely sensitive to small periods of daily strain applied axially within two weeks of fracture. There are boundaries of strain magnitude and force of application of applied movement that, if exceeded, inhibit the healing process. The application of appropriate applied strain to clinical tibial fractures at a time shortly after injury, when most patients would be very inactive, appears to enhance the healing process when using external skeletal fixation.
https://www.frontiersin.org/journals/surgery/articles/10.3389/fsurg.2021.749209/full
Non-union rate after tibial fractures remains high. Apart from largely uncontrollable biologic, injury, and patient-specific factors, the mechanical fracture environment is a key determinant of healing. Our aim was to establish a patient-specific simulation workflow to determine the mechanical fracture environment and allow for an estimation of its healing potential. In a referred patient with failed nail-osteosynthesis after tibial-shaft fracture exchange nailing was performed. Post-operative CT-scans were used to construct a three-dimensional model of the treatment situation in an image processing and computer-aided design system. Resulting forces, computed in a simulation-driven workflow based on patient monitoring and motion capturing were used to simulate the mechanical fracture environment before and after exchange nailing. Implant stresses for the initial and revision situation, as well as interfragmentary movement, resulting hydrostatic, and octahedral shear strain were calculated and compared to the clinical course. The simulation model was able to adequately predict hardware stresses in the initial situation where mechanical implant failure occurred. Furthermore, hydrostatic and octahedral shear strain of the revision situation were calculated to be within published healing boundaries—accordingly the fracture healed uneventfully. Our workflow is able to determine the mechanical environment of a fracture fixation, calculate implant stresses, interfragmentary movement, and the resulting strain. Critical mechanical boundary conditions for fracture healing can be determined in relation to individual loading parameters. Based on this individualized treatment recommendations during the early post-operative phase in lower leg fractures are possible in order to prevent implant failure and non-union development.
Introduction: Despite current clinical advances diaphyseal tibial fractures are associated with delayed- and non-union rates of over 10% (1–3). The development of a healing delay is dependent on many factors that often cannot be adequately influenced once the fracture has occurred (4). Of high significance in aseptic cases are vascularity and mechanical fracture environment (5). To determine the relevant mechanical influences on fracture healing numerical modeling and computer simulation has gained increasing interest (6). Based on the initial ideas of Pauwels, Wolff, Perren, and Frost ever more precise mechanical fracture environment boundary conditions for influencing tissue differentiation can now be given (7–12). Despite the differences between the models owed in part to the specifics of the simulations and input characteristics, these approaches and their experimental validation in animal research underscore the great importance of the mechanical environment for fracture healing. Two of the most relevant parameters to determine tissue differentiation during the course of fracture healing are interfragmentary shear strain and tensile or compressive volumetric strain (13, 14). While the clinical use of these simulated parameters has been shown in the case of a patient treated with an external fixator (15), these boundary conditions for hydrostatic pressure and volumetric fracture strain have yet to be applied to a clinical case with internal osteosynthesis. Despite its theoretical relevance especially in cases where failed fracture healing and failure of implant material point toward a high mechanical influence this has not been performed. Current clinical management is largely based on general treatment principles and surgeon experience depending on the applied hardware.
The aim of this study was, thus, to establish a simulation workflow based on clinical imaging data to (1) determine the pre- and post-treatment mechanical fracture environment in a tibial fracture revision case, (2) simulate the associated volumetric strain and octahedral shear strain resulting from patient weight-bearing, and (3) provide a clinical proof-of-concept over the treatment course.
Materials and Methods: A 55-year old, female patient (height 152 cm, weight 73 kg) was treated for an open distal tibial shaft fracture (Figures 1A,B) with debridement, temporary external fixation, negative pressure wound therapy, unreamed intramedullary tibial nailing (8 mm diameter) (Figures 1C,D) and MESH graft skin closure at an external institution within the span of 4 weeks. Immediate post-operative full weight-bearing was ordered. Approximately 7 weeks after the tibial nail procedure was performed and without further trauma the patient suffered from an implant failure and refracture of the initial situation (Figures 1E,F). She was then referred for treatment to our institution, where after an initial hardware removal, temporary external fixation and histological and microbiological exclusion of infection, a reamed nailing procedure (9 mm nail diameter) and fibular plate osteosynthesis was performed (Figures 1G,H). Again, immediate post-operative full weight-bearing was performed and reached within the inpatient stay, as controlled with plantar pressure measurements (16). To estimate the non-union risk of the patient the Non-Union Risk Development (NURD) Score was calculated as 7 (17). Furthermore, the patient suffered from a severe Neurofibromatosis, that if counted as a chronic condition would increase the NURD score further to 9. The patient consented to the study. Only routine imaging data was used and the study was approved by the local ethics committee.